Recombinant: Superior lot-to-lot consistency, continuous supply, and animal-free manufacturing.
Myeloperoxidase (E1E7I) XP® Rabbit mAb (Alexa Fluor® 647 Conjugate) #17646
Filter:
- IF
- F
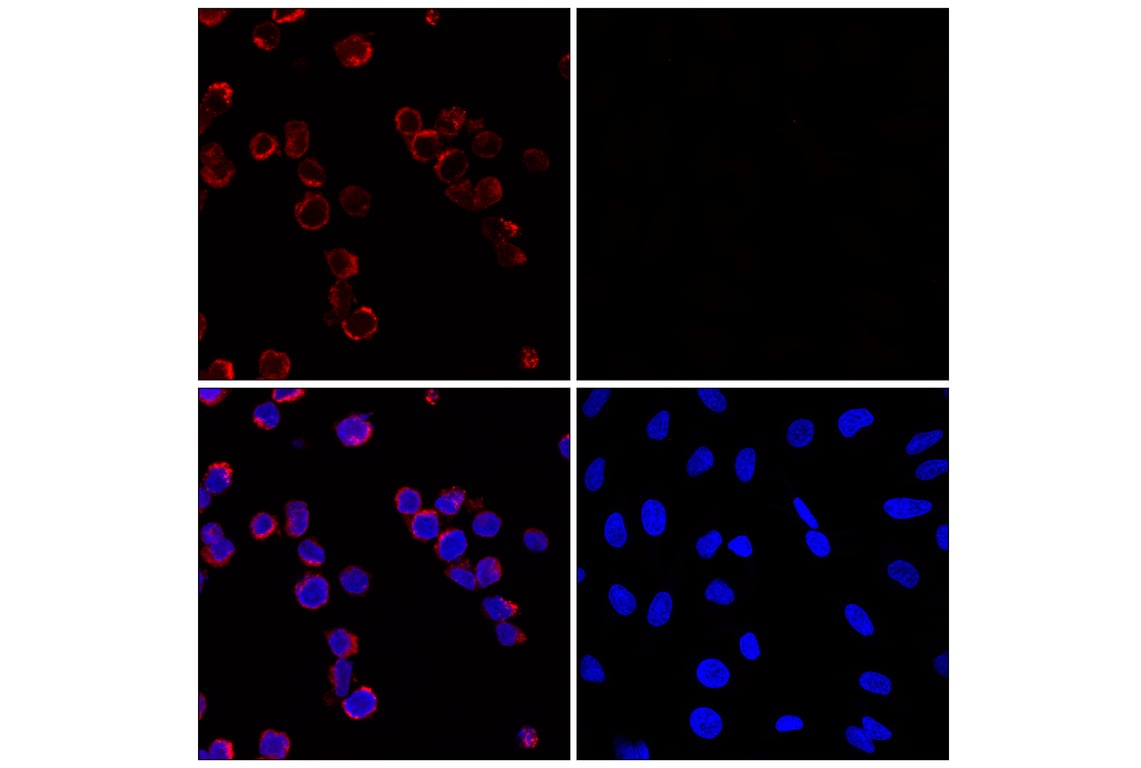
Supporting Data
| REACTIVITY | H |
| SENSITIVITY | Endogenous |
| MW (kDa) | |
| Source/Isotype | Rabbit IgG |
Application Key:
- IF-Immunofluorescence
- F-Flow Cytometry
Species Cross-Reactivity Key:
- H-Human
- Related Products
Product Information
Product Description
This Cell Signaling Technology antibody is conjugated to Alexa Fluor® 647 fluorescent dye under optimal conditions and tested in-house for direct flow cytometric and immunofluorescent analysis in human cells. This antibody conjugate is expected to exhibit the same cross-reactivity as the unconjugated Myeloperoxidase (E1E7I) XP® Rabbit mAb #14569.
Product Usage Information
| Application | Dilution |
|---|---|
| Immunofluorescence (Immunocytochemistry) | 1:50 |
| Flow Cytometry (Fixed/Permeabilized) | 1:50 |
Storage
Supplied in PBS (pH 7.2), less than 0.1% sodium azide, and 2 mg/mL BSA. Store at 4°C. Do not aliquot the antibody. Protect from light. Do not freeze.
Protocol
Specificity / Sensitivity
Myeloperoxidase (E1E7I) XP® Rabbit mAb (Alexa Fluor® 647 Conjugate) recognizes endogenous levels of total myeloperoxidase protein. This antibody recognizes the full-length and heavy chain subunits of human myeloperoxidase protein.
Species Reactivity:
Human
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Pro734 of human myeloperoxidase protein.
Background
Myeloperoxidase (MPO) is a peroxidase enzyme that is part of the host defense system of polymorphonuclear leukocytes (reviewed in 1). The gene for MPO was cloned independently from several laboratories (2-5). A decrease in MPO expression was noticed upon differentiation of HL-60 cells (5). MPO catalyzes the reaction of hydrogen peroxide and chloride (or other halides) to produce hypochlorous acid and other potent antimicrobial oxidants. Knockout mice of MPO are impaired in clearing select microbial infections (6). Processing of mature MPO from an initial 80-90 kDa translation product involves insertion of a heme moiety, glycosylation, and proteolytic cleavage. The mature protein is a tetramer of two heavy chains (60 kDa) and two light chains (12 kDa). It is abundantly expressed in neutrophils and monocytes and secreted during their activation. Heightened MPO levels have been associated with tissue damage and a number of pathological conditions (1).
- Klebanoff, S.J. (2005) J Leukoc Biol 77, 598-625.
- Chang, K.S. et al. (1986) Blood 68, 1411-4.
- Yamada, M. et al. (1987) Arch Biochem Biophys 255, 147-55.
- Morishita, K. et al. (1987) J Biol Chem 262, 3844-51.
- Weil, S.C. et al. (1987) Proc Natl Acad Sci USA 84, 2057-61.
- Aratani, Y. et al. (2002) J Infect Dis 185, 1833-7.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not for Use in Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
Alexa Fluor is a registered trademark of Life Technologies Corporation.
XP is a registered trademark of Cell Signaling Technology, Inc.
This product is provided under an intellectual property license from Life Technologies Corporation. The transfer of this product is conditioned on the buyer using the purchased product solely in research conducted by the buyer, excluding contract research or any fee for service research, and the buyer must not (1) use this product or its components for (a) diagnostic, therapeutic or prophylactic purposes; (b) testing, analysis or screening services, or information in return for compensation on a per-test basis; or (c) manufacturing or quality assurance or quality control, and/or (2) sell or transfer this product or its components for resale, whether or not resold for use in research. For information on purchasing a license to this product for purposes other than as described above, contact Life Technologies Corporation, 5791 Van Allen Way, Carlsbad, CA 92008 USA or outlicensing@lifetech.com.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.