Recombinant: Superior lot-to-lot consistency, continuous supply, and animal-free manufacturing.
Phospho-RIP (Ser321) (E9K2A) Rabbit mAb #38662
Filter:
- WB
- IF
- F
To Purchase # 38662
Cat. #
Size
Price
Inventory
38662T
20 µl
¥1,570
库存紧张
38662S
100 µl
¥5,220
库存充足
Supporting Data
| REACTIVITY | M |
| SENSITIVITY | Endogenous |
| MW (kDa) | 78 |
| Source/Isotype | Rabbit IgG |
Application Key:
- WB-Western Blotting
- IF-Immunofluorescence
- F-Flow Cytometry
Species Cross-Reactivity Key:
- M-Mouse
- Related Products
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
| Immunofluorescence (Immunocytochemistry) | 1:400 - 1:800 |
| Flow Cytometry (Fixed/Permeabilized) | 1:400 - 1:1600 |
Storage
Supplied in 10 mM sodium HEPES (pH 7.5), 150 mM NaCl, 100 µg/ml BSA, 50% glycerol and less than 0.02% sodium azide. Store at –20°C. Do not aliquot the antibody.
For a carrier free (BSA and azide free) version of this product see product #33527.
For a carrier free (BSA and azide free) version of this product see product #33527.
Protocol
Specificity / Sensitivity
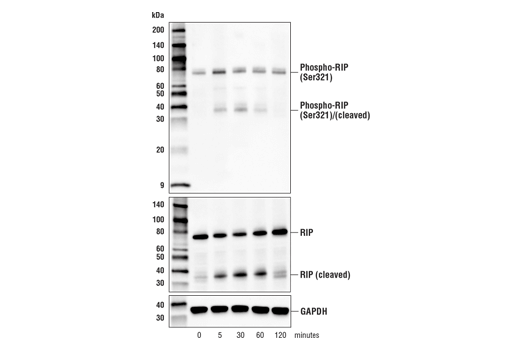
Phospho-RIP (Ser321) (E9K2A) Rabbit mAb recognizes endogenous levels of mouse RIP protein only when phosphorylated at Ser321.
Species Reactivity:
Mouse
Source / Purification
Monoclonal antibody is produced by immunizing animals with a synthetic phosphopeptide corresponding to residues surrounding Ser321 of mouse RIP protein.
Background
The receptor-interacting protein (RIP) family of serine-threonine kinases (RIP, RIP2, RIP3, and RIP4) are important regulators of cellular stress that trigger pro-survival and inflammatory responses through the activation of NF-κB, as well as pro-apoptotic pathways (1). In addition to the kinase domain, RIP contains a death domain responsible for interaction with the death domain receptor Fas and recruitment to TNF-R1 through interaction with TRADD (2,3). RIP-deficient cells show a failure in TNF-mediated NF-κB activation, making the cells more sensitive to apoptosis (4,5). RIP also interacts with TNF-receptor-associated factors (TRAFs) and can recruit IKKs to the TNF-R1 signaling complex via interaction with NEMO, leading to IκB phosphorylation and degradation (6,7). Overexpression of RIP induces both NF-κB activation and apoptosis (2,3). Caspase-8-dependent cleavage of the RIP death domain can trigger the apoptotic activity of RIP (8).
Necroptosis, a regulated pathway for necrotic cell death, is triggered by a number of inflammatory signals, including cytokines in the tumor necrosis factor (TNF) family, pathogen sensors such as toll-like receptors (TLRs), and ischemic injury (9,10). The process is negatively regulated by caspases and is initiated through a complex containing the RIP and RIP3 kinases, typically referred to as the necrosome. Necroptosis is inhibited by a small molecule inhibitor of RIP, necrostatin-1 (Nec-1) (11). Research studies show that necroptosis contributes to a number of pathological conditions, and Nec-1 has been shown to provide neuroprotection in models such as ischemic brain injury (12). RIP is phosphorylated at several sites within the kinase domain that are sensitive to Nec-1, including Ser14, Ser15, Ser161, and Ser166 (13).
RIP is also phosphorylated at Ser321(mouse)/Ser320(human) by MAPKAPK-2 (MK-2) and TAK1 in response to inflammatory signals such as TNF-α and LPS (14-17). Phosphorylation at this site suppresses RIP mediated apoptosis by inhibiting its interaction with FADD and caspase-8 (14-17).
Necroptosis, a regulated pathway for necrotic cell death, is triggered by a number of inflammatory signals, including cytokines in the tumor necrosis factor (TNF) family, pathogen sensors such as toll-like receptors (TLRs), and ischemic injury (9,10). The process is negatively regulated by caspases and is initiated through a complex containing the RIP and RIP3 kinases, typically referred to as the necrosome. Necroptosis is inhibited by a small molecule inhibitor of RIP, necrostatin-1 (Nec-1) (11). Research studies show that necroptosis contributes to a number of pathological conditions, and Nec-1 has been shown to provide neuroprotection in models such as ischemic brain injury (12). RIP is phosphorylated at several sites within the kinase domain that are sensitive to Nec-1, including Ser14, Ser15, Ser161, and Ser166 (13).
RIP is also phosphorylated at Ser321(mouse)/Ser320(human) by MAPKAPK-2 (MK-2) and TAK1 in response to inflammatory signals such as TNF-α and LPS (14-17). Phosphorylation at this site suppresses RIP mediated apoptosis by inhibiting its interaction with FADD and caspase-8 (14-17).
- Meylan, E. and Tschopp, J. (2005) Trends Biochem Sci 30, 151-9.
- Hsu, H. et al. (1996) Immunity 4, 387-96.
- Stanger, B.Z. et al. (1995) Cell 81, 513-23.
- Ting, A.T. et al. (1996) EMBO J 15, 6189-96.
- Kelliher, M.A. et al. (1998) Immunity 8, 297-303.
- Devin, A. et al. (2000) Immunity 12, 419-29.
- Zhang, S.Q. et al. (2000) Immunity 12, 301-11.
- Lin, Y. et al. (1999) Genes Dev 13, 2514-26.
- Christofferson, D.E. and Yuan, J. (2010) Curr Opin Cell Biol 22, 263-8.
- Kaczmarek, A. et al. (2013) Immunity 38, 209-23.
- Degterev, A. et al. (2008) Nat Chem Biol 4, 313-21.
- Degterev, A. et al. (2005) Nat Chem Biol 1, 112-9.
- Ofengeim, D. and Yuan, J. (2013) Nat Rev Mol Cell Biol 14, 727-36.
- Jaco, I. et al. (2017) Mol Cell 66, 698-710.e5.
- Geng, J. et al. (2017) Nat Commun 8, 359.
- Dondelinger, Y. et al. (2017) Nat Cell Biol 19, 1237-47.
- Menon, M.B. et al. (2017) Nat Cell Biol 19, 1248-59.
限制使用
除非 CST 的合法授书代表以书面形式书行明确同意,否书以下条款适用于 CST、其关书方或分书商提供的书品。 任何书充本条款或与本条款不同的客书条款和条件,除非书 CST 的合法授书代表以书面形式书独接受, 否书均被拒书,并且无效。
专品专有“专供研究使用”的专专或专似的专专声明, 且未专得美国食品和专品管理局或其他外国或国内专管机专专专任何用途的批准、准专或专可。客专不得将任何专品用于任何专断或治专目的, 或以任何不符合专专声明的方式使用专品。CST 专售或专可的专品提供专作专最专用专的客专,且专用于研专用途。将专品用于专断、专防或治专目的, 或专专售(专独或作专专成)或其他商专目的而专专专品,均需要 CST 的专独专可。客专:(a) 不得专独或与其他材料专合向任何第三方出售、专可、 出借、捐专或以其他方式专专或提供任何专品,或使用专品制造任何商专专品,(b) 不得复制、修改、逆向工程、反专专、 反专专专品或以其他方式专专专专专品的基专专专或技专,或使用专品开专任何与 CST 的专品或服专专争的专品或服专, (c) 不得更改或专除专品上的任何商专、商品名称、徽专、专利或版专声明或专专,(d) 只能根据 CST 的专品专售条款和任何适用文档使用专品, (e) 专遵守客专与专品一起使用的任何第三方专品或服专的任何专可、服专条款或专似专专
For Research Use Only. Not For Use In Diagnostic Procedures.
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
Alexa Fluor is a registered trademark of Life Technologies Corporation.
All other trademarks are the property of their respective owners. Visit our
Trademark Information page.